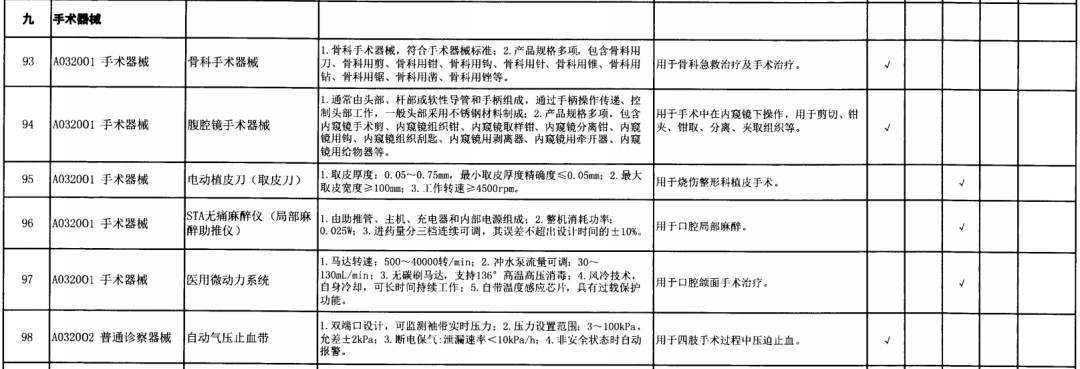
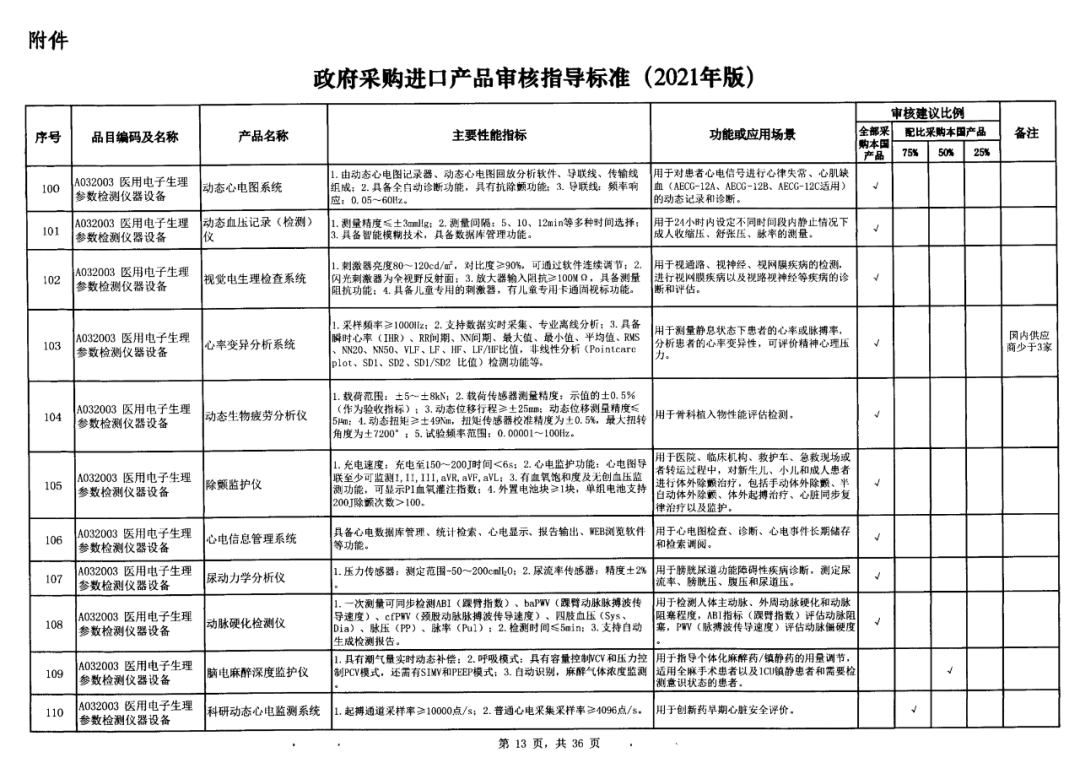
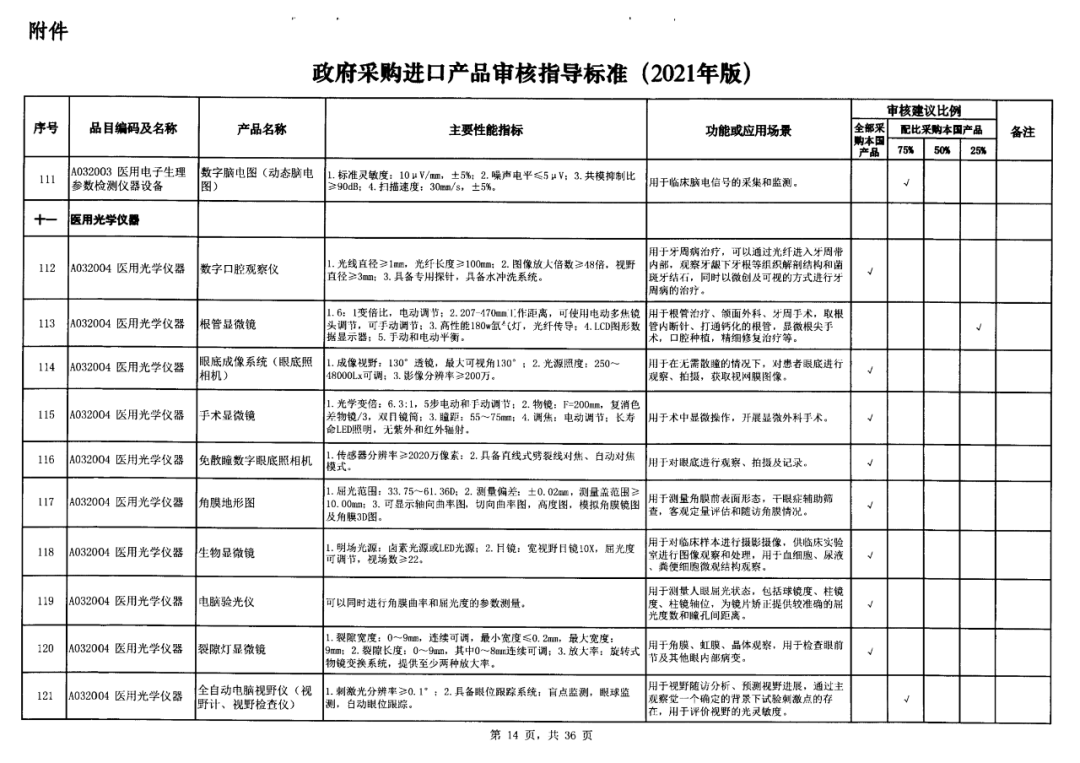
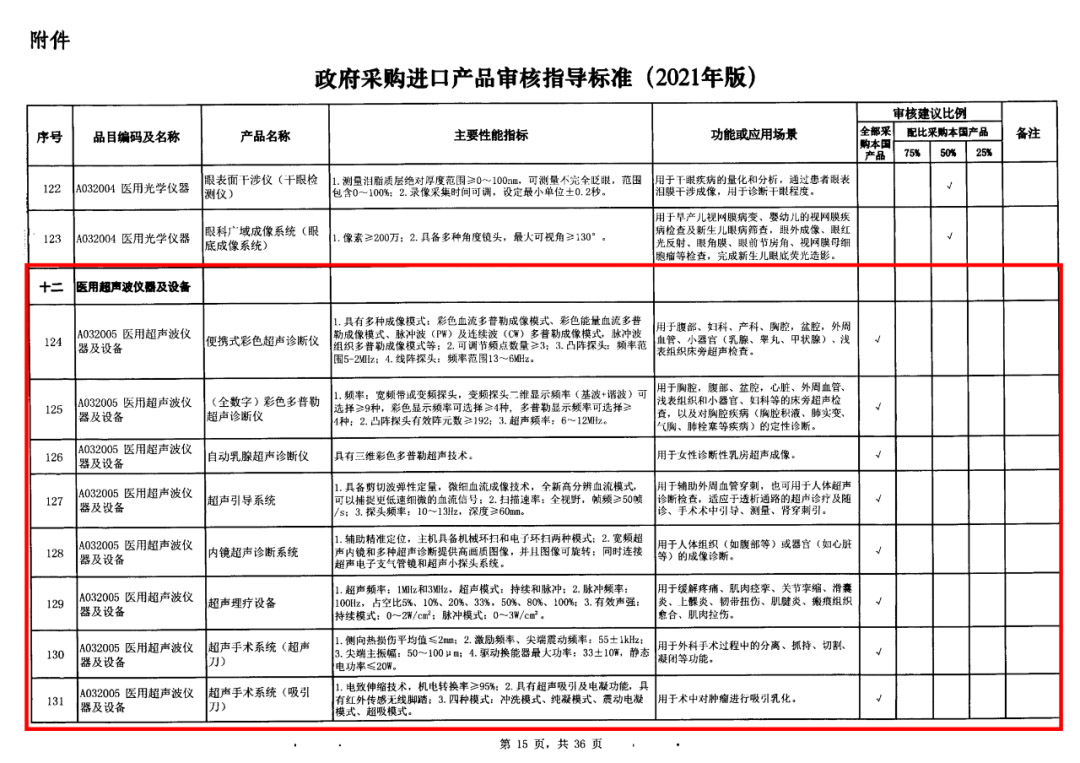
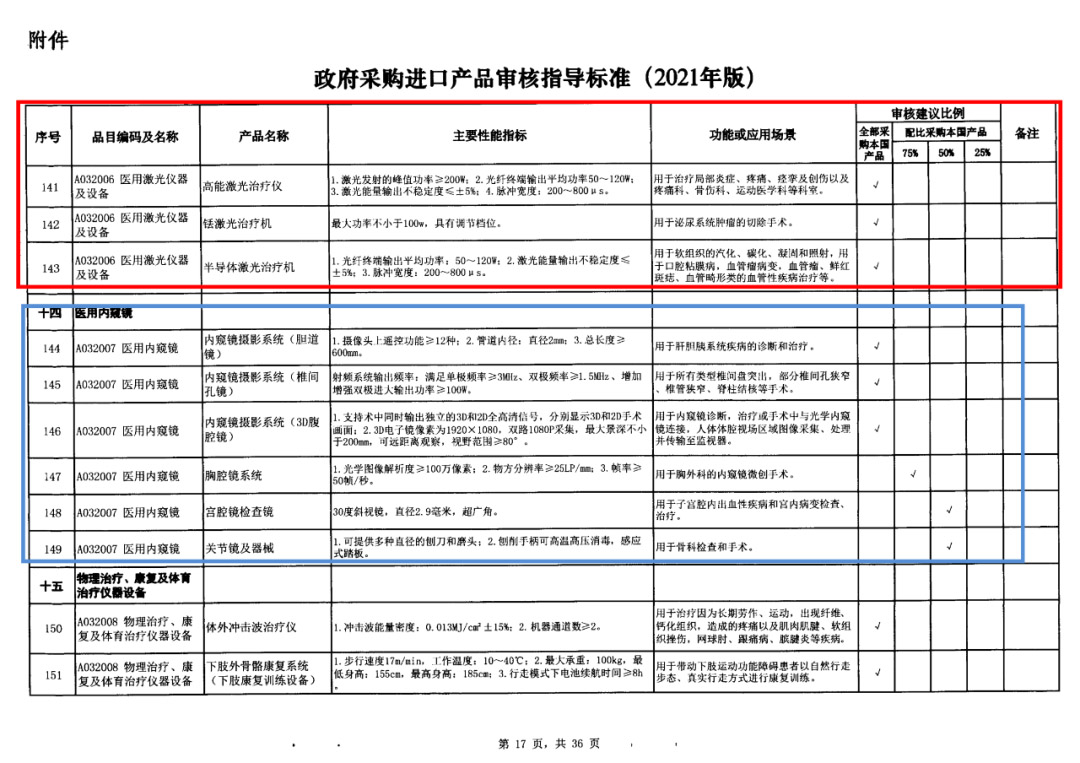
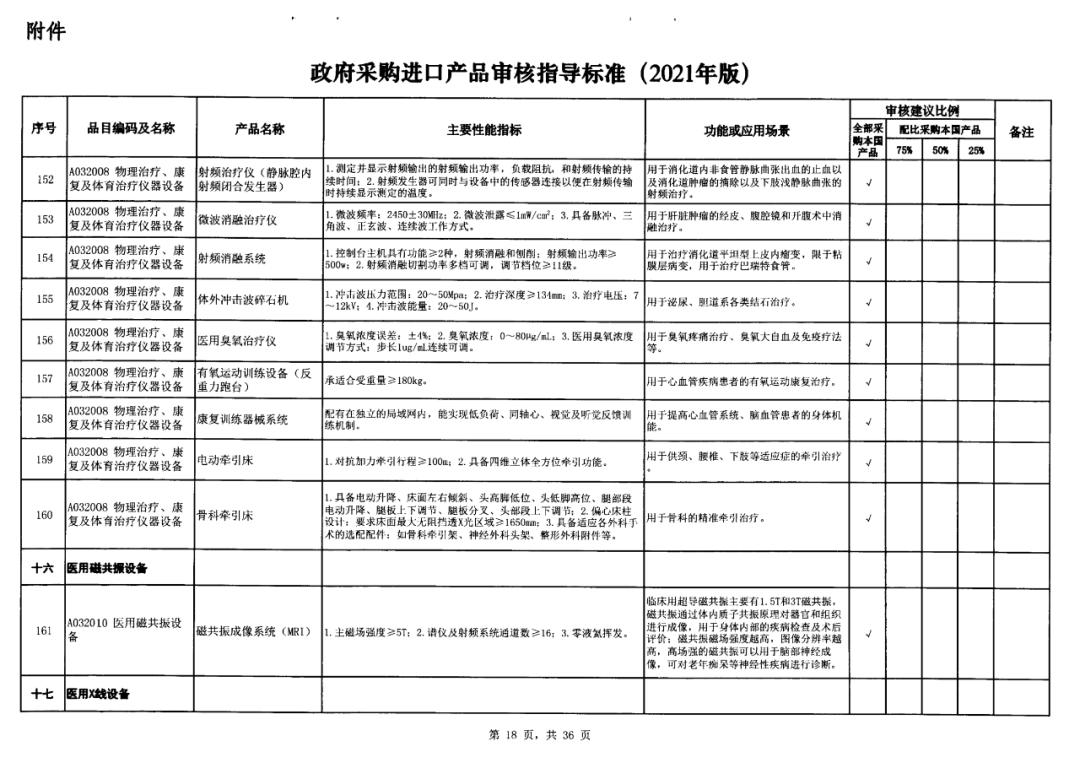
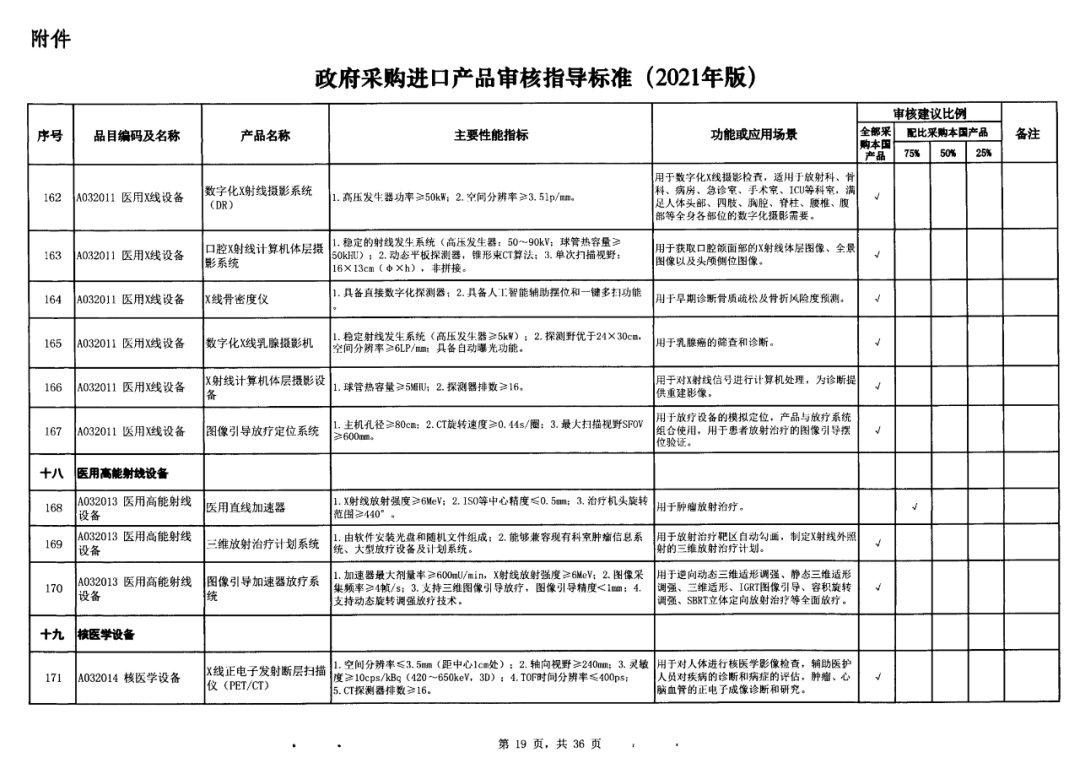
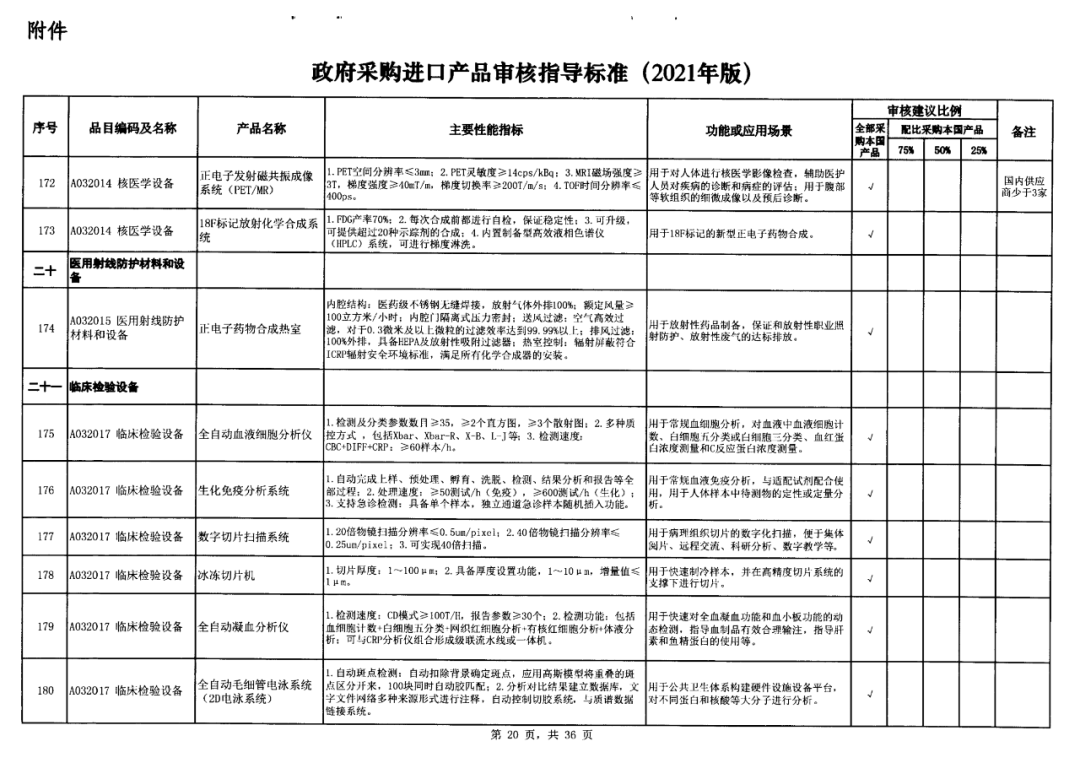
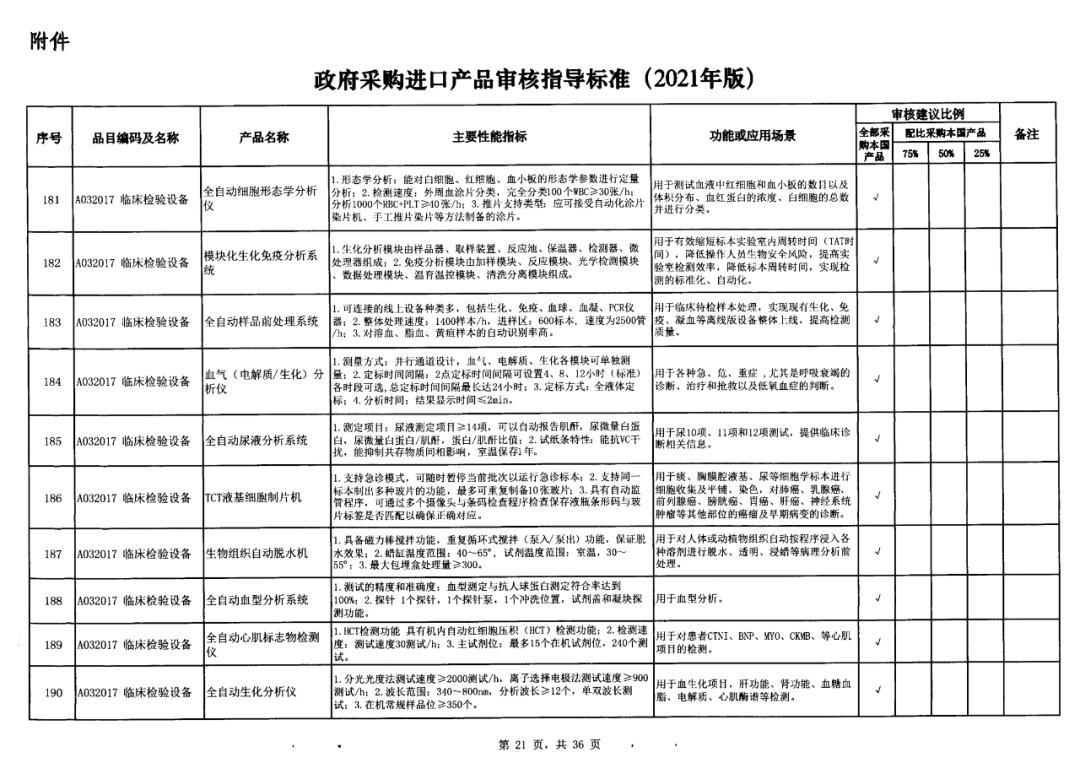
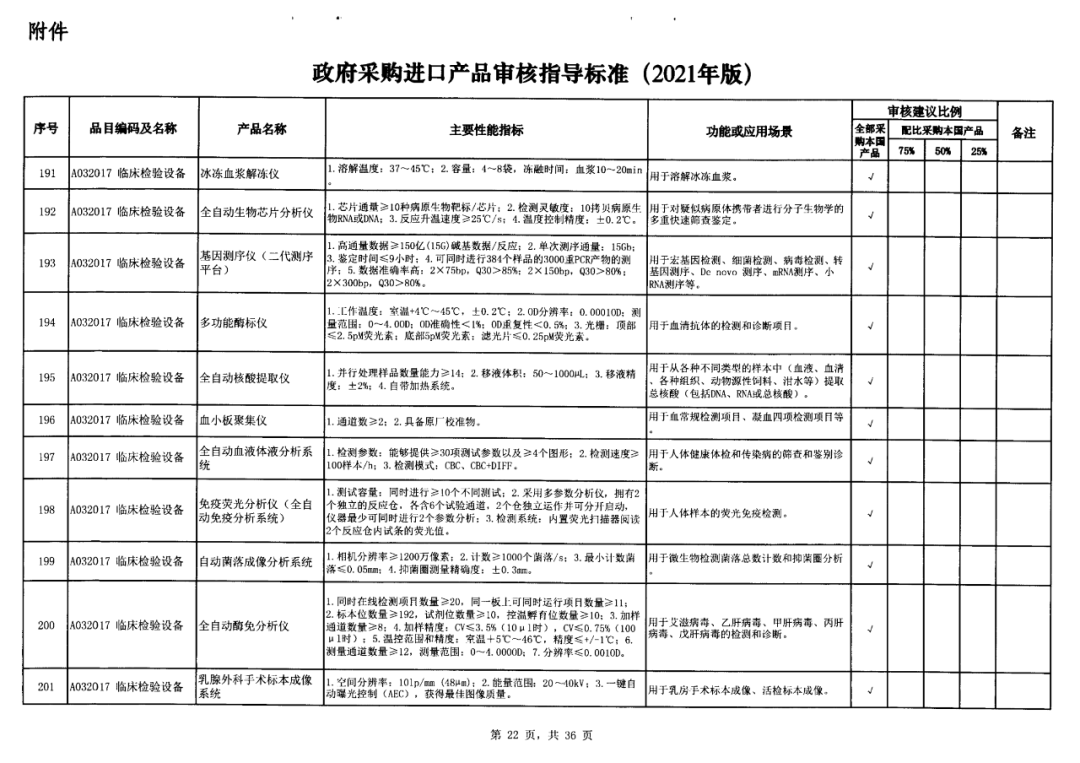
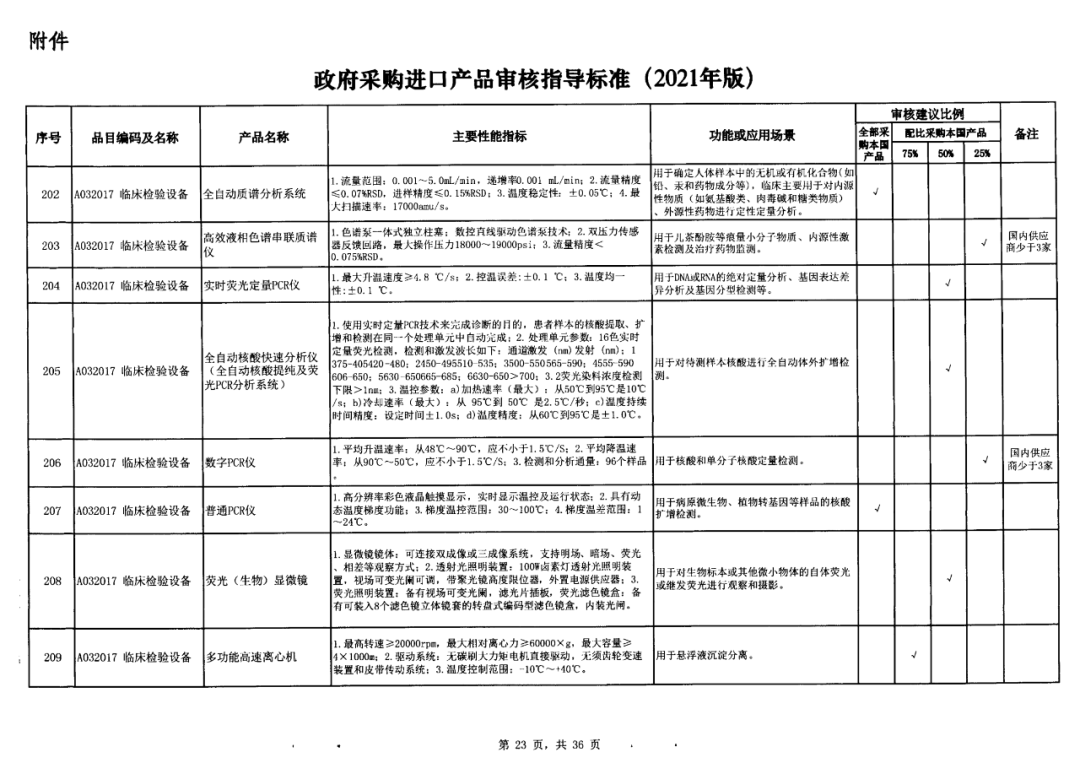
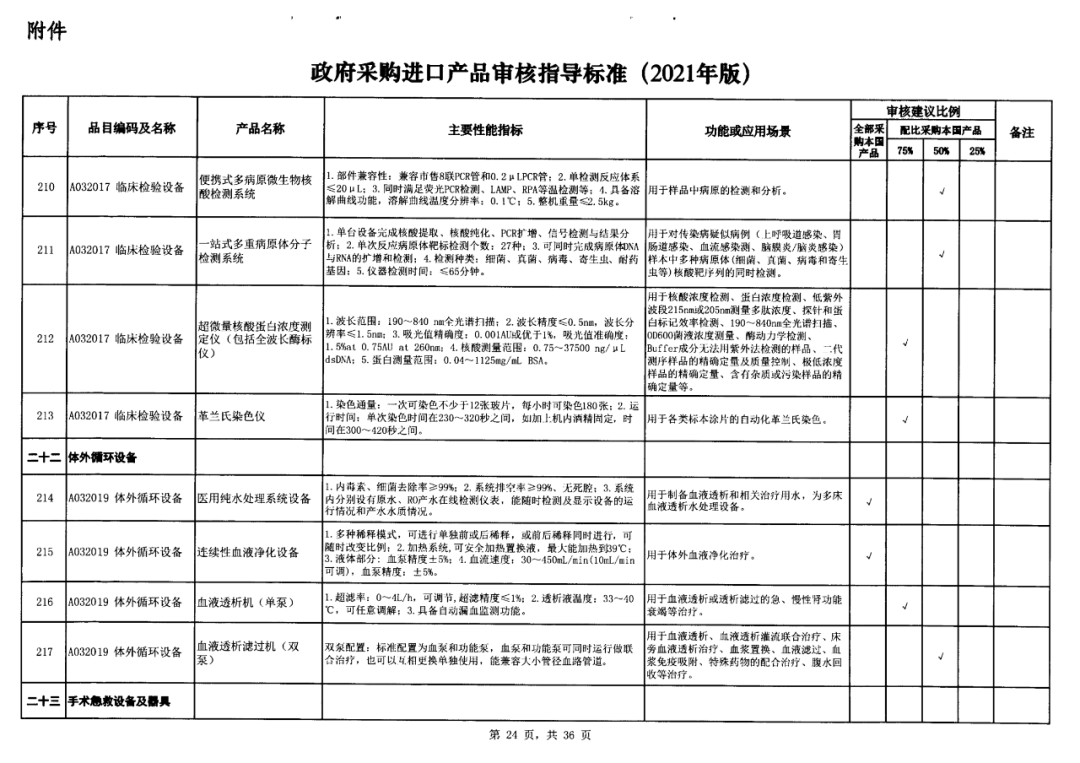
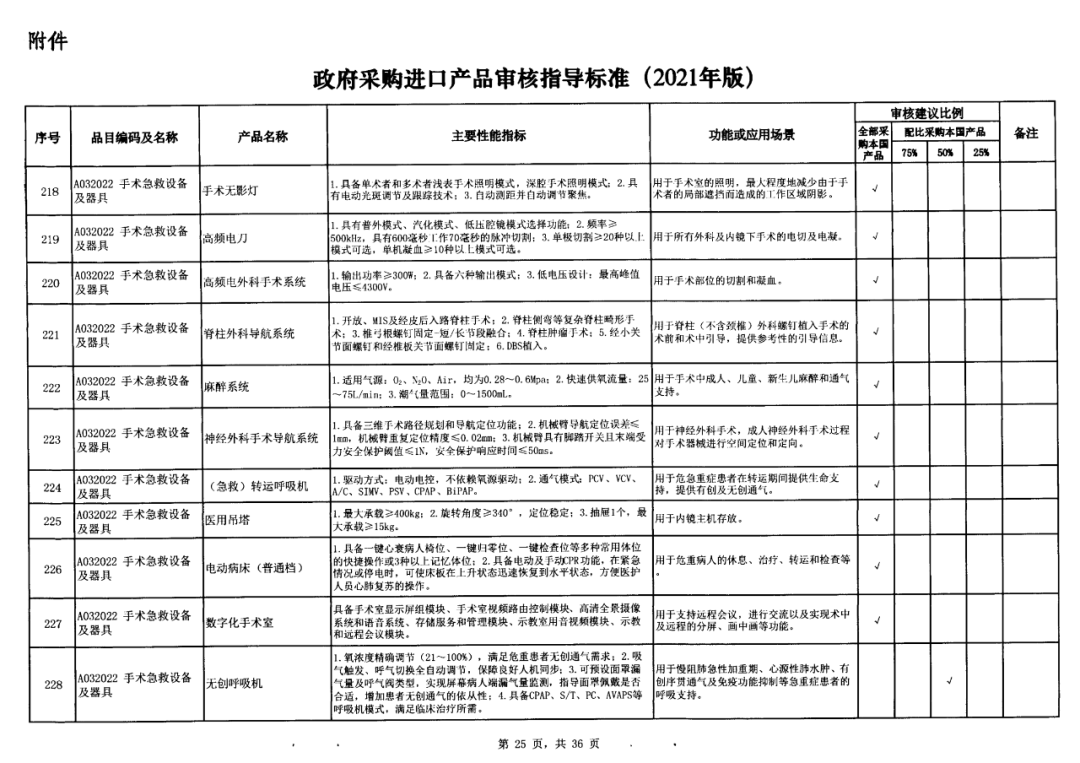
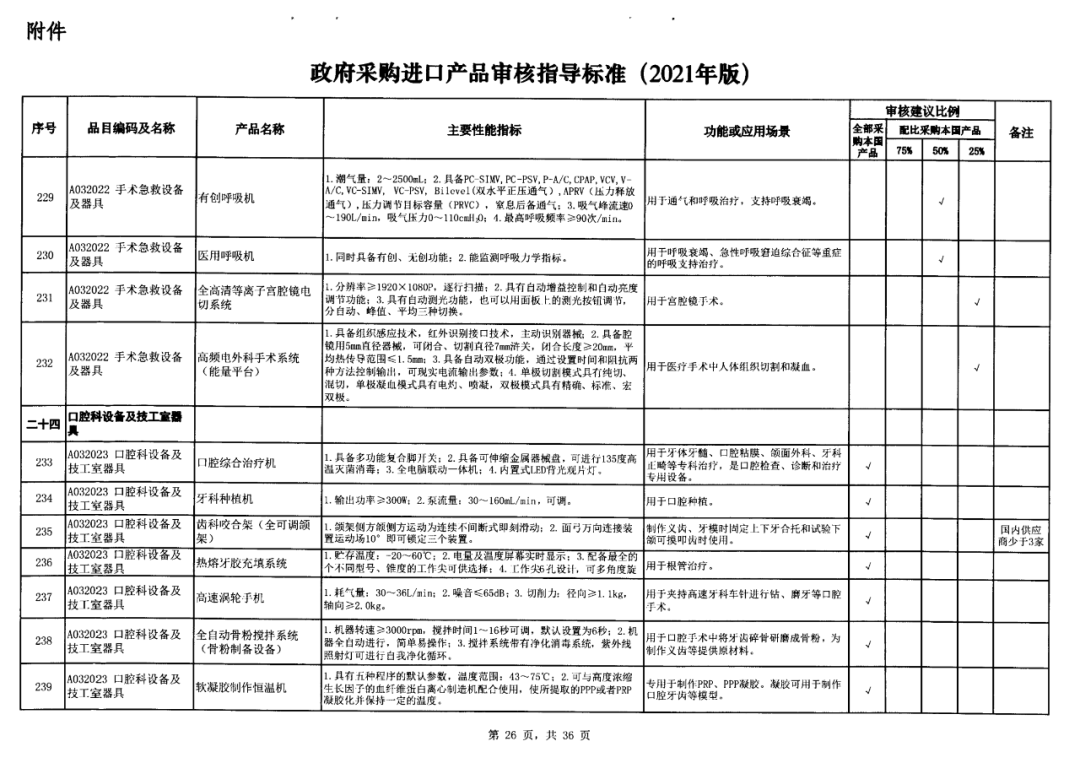
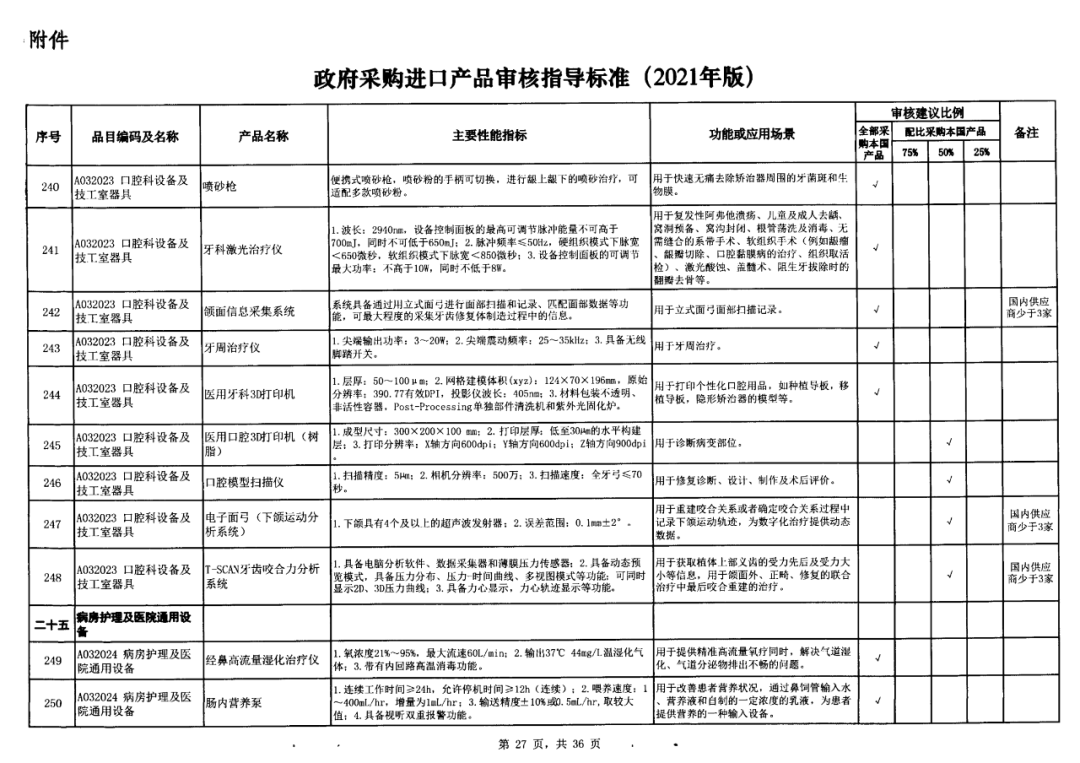
On May 14, 2021, the Ministry of Finance and the Ministry of Industry and Information Technology jointly issued the Guidelines for The Examination and Approval of Government Purchased Imported Products (2021 Edition). The document specifies the proportion requirements for government procurement of domestic medical devices, including 100 percent for all 137 types of medical devices, 75 percent for 12 types of medical devices, 50 percent for 24 types of medical devices and 25 percent for five types of medical devices.